Abstract
More than 170 subspecies are listed as threatened or endangered under the US Endangered Species Act. Most of these subspecies were described decades ago using relatively primitive taxonomic methods. The status quo in government agencies is to accept all named subspecies as taxonomically valid. The US Fish and Wildlife Service basically defaults to taxonomists working with specific groups of organisms, and as a result there is no single definition of subspecies across plants and animals. However, the Integrated Taxonomic Information System maintained by the US Fish and Wildlife Service makes judgments about the validity of subspecies without providing supporting evidence. We find that subspecies designations repeatedly lack empirical and theoretical consistency, ranging from arbitrary divisions of single character clines to strongly supported evolutionary units. Valid tests today usually entail molecular analyses of variation within and among populations, including tests for significant differences between samples of the putative endangered subspecies and its nearest geographic relatives. Evaluation of data gathered since subspecies were described suggests that about one-third are valid, one-third are not, and one-third have not been tested. Therefore, it should not be assumed that because a subspecies is listed, it is taxonomically valid. Some new molecular methods (next-generation sequencing) will find differences between most if not all geographically separated populations, and it remains to be seen how these populations, whether named as subspecies or not, will be adjudicated under the Endangered Species Act. It will also be useful to explore whether listed subspecies are ecologically significant. A unified scheme to prioritize listed entities on the basis of their level of taxonomic and ecological significance is yet to be proposed or accepted.
Introduction to the Endangered Species Act and Taxonomic Categories
The legislative basis for much of the conservation effort in the United States is the Endangered Species Act (ESA), passed in 1973 and modified in 1978, 1982, and 1988. Given its title, one might expect it to apply only to species, but in fact it also can be used to list subspecies and distinct population segments (vertebrates only) as threatened or endangered. Listing decisions usually come about when either a U.S. citizen or organization, or the Fish and Wildlife Service itself, determines that the population size of one of these taxonomic entities places it in danger of extinction (endangered) or in almost as much peril (threatened). The species or subspecies is placed on a list of candidate species, and the Fish and Wildlife Service is directed to use the best available scientific or commercial data in making a ruling as to whether the taxon merits listing as endangered or threatened. In this paper we examine the taxonomic category or rank of subspecies. In particular, we determine whether modern tests of subspecies limits have confirmed the validity of listed subspecies, most of which were described more than a half-century ago.
Taxonomic Background and the Concept of Subspecies
Taxonomy and Concepts of Species and Subspecies
Systematists, taxonomists, and evolutionary biologists have struggled to define the term species for a century and a half. The biological species concept recognizes a species as a diagnosable distinct population or group of populations that is reproductively isolated from other such populations or groups. Lineage concepts, on the other hand, such as the phylogenetic species concept, recognize diagnosable populations or groups of populations as species irrespective of whether they can hybridize with other such groups (Cracraft 1983). That is, diagnosable subspecies of biological species would more than likely be considered phylogenetic species (Barrowclough et al. 2016). Many other species concepts have been offered, including recent ones that search for congruence among multiple loci or character sets: so-called species-delimitation approaches (Malaney et al. 2017). Several methods automate the process of converting data on molecular variation with and between populations into estimates of numbers of species (e.g., Luo et al. 2018).
Below the level of the species, the biological species concept considers geographically differing populations to be subspecies when they are distinguishable by phenotypic or genotypic characters and are (or are presumed to be) capable of hybridizing. In general, most lineage views of species do not consider subspecies, because diagnostically distinct populations that are (or are presumed to be) reproductively compatible would be considered species (de Queiroz 2007). Thus, the debate about species concepts is somewhat tangential to determining the geographic limits of populations proposed for listing, although some authors (e.g., Haig et al. 2006; Mallet 2007) have confused the relationship between species and subspecies concepts and the relevance of these concepts to conservation. Species are usually considered more important than the lower taxonomic ranks, and typically conservationists’ question is to list or not to list them, whereas with subspecies, the question is often whether they are taxonomically valid in the first place. See contrasting views of wolf (Canis lupus) species and subspecies taxonomy in which the same data were considered (National Academies of Sciences, Engineering, and Medicine 2019; Cronin et al. 2015).
What Exactly Is a Subspecies?
A subspecies is a formal taxonomic category that is specified by three Latin names: the genus name, the species name, and the subspecies name. Subspecies are described in different ways depending on the organism. Definitions of subspecies range from whatever a taxonomist says is valid to multi-character genetic and morphological assessments (Zink 1997). Some favor a rule in which 75% of individuals in a subspecies must be separable from 99% in another subspecies—clearly an arbitrary standard. Taylor et al. (2017) suggest that “a subspecies is a population, or collection of populations, that appears to be a separately evolving lineage with discontinuities resulting from geography, ecological specialization, or other forces that restrict gene flow to the point that the population or collection of populations is diagnosably distinct.” For butterflies, Braby et al. (2012) concluded that “definition of subspecies be restricted to extant animal groups that comprise evolving populations representing partially isolated lineages of a species that are allopatric, phenotypically distinct, and have at least one fixed diagnosable character state, and that these character differences are (or are assumed to be) correlated with evolutionary independence according to population genetic structure.” We believe that this definition provides sufficient criteria for recognizing a subspecies as valid and potentially qualified to be listed under the ESA should it become threatened or endangered. However, the subspecies category has been used in different ways by taxonomists in the same groups and in different groups. That is, the expectation from the definition proposed by Taylor et al. (2017) is evolutionary independence of subspecies, whereas the 75% rule carries no such connotation. This is a fundamentally important difference. The lack of consistency is presumably the reason the US Fish and Wildlife Service (USFWS) has no set definition of subspecies, instead relying on peer-reviewed literature that reflects the views of taxonomists in different groups. It would be beneficial to all involved if the USFWS would at least provide a minimal set of criteria for determining whether a subspecies is listable.
Historical Views on Subspecies
Issues surrounding subspecies have drawn considerable attention. In 1992, National Academy scientist John C. Avise wrote,
Taxonomic assignments inevitably shape perceptions of biological diversity. Therefore, it is disconcerting that many subspecies and species descriptions trace to very limited information, often gathered in the last century, on the distributions of a small number of (usually morphological) traits with unknown genetic basis. Yet once a Latin binomial or trinomial is in the literature, the group of organisms to which it refers almost automatically assumes an aura of reality that may or may not be commensurate with its true evolutionary distinctiveness. Given the overriding importance of taxonomy on biodiversity recognition and management, increased attention should be devoted to taxonomic assessments (from molecular as well as other data). . . . Population managers should therefore keep an open mind regarding taxonomic realignments, particularly for populations within the majority of species that have remained poorly studied.
Avise’s sentiment could not be truer today, and it is not unique. In 1953, Wilson and Brown wrote, “The subspecies concept is the most critical and disorderly area of modern systematic theory.” Mayr (1970) wrote, “This concept of the subspecies is fallacious. Species are not composites of uniform subtypes—subspecies—but consist of an almost infinite number of local populations, each in turn (in sexual species) consisting of genetically different individuals. . . . The better the geographic variation of a species is known, the more difficult it becomes to delimit subspecies and the more obvious it becomes that many such delimitations are quite arbitrary.” Remsen (2005) commented on avian subspecies, “Is it any wonder, therefore, that the roster of formal subspecies, most described before the advent of statistical methods in ornithology, contains many names that refer only to arbitrary points on clines, average differences between populations, or zones of intergradation . . . rather than to discrete entities?” Barrowclough (1982) wrote, “It seems curious that qualitative examination of color or a few skin measurements of a few specimens, often without statistical tests for clines or without adequate sampling of intermediate geographic areas, frequently results in trinomials, while the authors of large, quantitative studies frequently avoid them. This strongly suggests to me that most subspecies are not to be taken too seriously.” Zink (2004) wrote, “A massive reorganization of classifications is required so that the lowest ranks, be they species or subspecies, reflect evolutionary diversity. Until such reorganization is accomplished, the subspecies rank will continue to hinder progress in taxonomy, evolutionary studies and especially conservation.” Lastly, Burbrink et al. (in press) state succinctly: “We argue that the use of subspecies is indefensible on philosophical and empirical grounds.”
Of course, there are (at least) two sides to all issues. Patten and Remsen (2017) discount the concerns of Wilson and Brown (1953): “Wilson and Brown’s point of view was easy to understand because in that era many subspecies were being described for trivial or inconsistent reasons and with no little effort to establish standards.” Actually, Patten and Remsen (2017) inadvertently cast suspicion on subspecies listed under the ESA because, as we note below, 137 of the 176 subspecies (80%) were described before Wilson and Brown’s paper appeared. As we also note below, many avian biologists support the recognition of subspecies, both as a tool for evolutionary research (Winker 2010) and for use in conservation decisions.
Nonetheless, it is difficult to state the problem any better than Avise did three decades ago. What Avise did not accomplish, however, was to provide the details that support his argument. That is, every taxonomic description of a new subspecies provides some kind of supporting data for its recognition and significance, including the number of specimens examined, the number and location of sampling sites and the extent of the range examined, the number and types of characters, and statistical methods for analyzing character data. The pertinent question is whether these descriptions of subspecies, many adduced with century-old “technology,” are acceptable at face value today without assessments using modern technology. It seems obvious that conservationists should evaluate whether new technologies tend to validate or confirm the reality of a subspecies. We provide such an evaluation below.
Subspecies Studies: Some Good and Some Bad
Rigorous application of subspecies names has not historically been the status quo. Consider the Rio Grande subspecies (Meleagris gallopavo intermedia) of the North American wild turkey. The subspecific part of the scientific name, intermedia, was justified by the author (Sennett 1879) because it was his opinion that the turkey’s appearance was “intermediate” between two other subspecies. Exactly where it starts and stops being intermediate was not documented by the author, which understandably confounds placement of subspecies boundaries. There can be no better example of Mayr’s (1970) caricature of some subspecies than this turkey subspecies. Unless, perhaps, it is the subspecies of white-tailed deer(Odocoileus virginianus leucurus) from the Columbia River area that was described on the basis of a single specimen, which was later consumed by the hunter who harvested it.
There are several examples in which subspecies correspond to genetically or morphologically defined units that have experienced evolutionarily independent histories and therefore qualify for listing under the ESA. For example, the spotted owl (Strix occidentalis) has three subspecies: the northern spotted owl (S. o. caurina), California spotted owl (S. o. occidentalis), and Mexican spotted owl (S. o. lucida). Barrowclough et al. (2006; 2011) show that each subspecies is genetically distinct, with a narrow hybrid zone between northern and California spotted owls. Given their evolutionary independence, these subspecies are worthy of separate conservation status, and one could easily consider each one a species. Vázquez-Miranda et al. (2019) show that subspecies of the LeConte’s thrasher (Toxostoma lecontei) found in the Vizcaino Desert of Baja California, and populations to the north, are genetically distinct and qualify as units worthy of conservation status. Catanach et al. (2021) provide a textbook example of how a subspecies should be tested with modern methods. They examined the status of the hawk Accipter straitus venator from Puerto Rico using ultra-conserved elements (nuclear DNA), mitochondrial DNA (mtDNA), and morphology. Their study shows that the specimens from the island formed a discrete genetic cluster, and in fact they suggest A. s. venator be raised to a full species. The fact that the population is on an island reduces the chances that there will be clinal variation as a result of gene flow linking adjacent populations, as is often the case with widely distributed species on a continent.
These are three examples of subspecies that would be of interest to evolutionary biologists seeking to explain how and when differentiation arose. They are, however, in the minority of described subspecies. At the other end of the continuum, Benedict et al. (2019) used molecular methods to test subspecies limits in Heermann’s kangaroo rat (Dipodomys heermanni), including the federally listed D. h. morroensis, and concluded “that D. h. morroensis is not genetically or morphologically distinct, with no support for monophyly of D. h. morroensis in any of the molecular analyses.” (Monophyly would be the situation in which all populations trace to a single common ancestor.) Other examples are provided in the supplementary information.
Integrated Taxonomic Information System
The USFWS maintains a website called the Integrated Taxonomic Information System (ITIS) where subspecies are classified as valid or invalid.1ITIS: Integrated Taxonomic Information System (home page), accessed May 31, 2022, https://www.itis.gov/servlet/SingleRpt/SingleRpt. According to the USFWS, “ITIS taxonomy is based on the latest scientific consensus available, and is provided as a general reference source for interested parties.” The page refers to some publications, but the evidence for subspecies validity is not given, and the evaluations cannot be verified. When there is no critical evaluation of a subspecies, the status quo is assumed and the subspecies is classified as valid as long as it is included in one or more taxonomic checklists.
Scientific Societies and Their Views on Subspecies
Taxonomists struggle to come up with consistent ways to delimit subspecies (Taylor et al. 2017). In fact, several authoritative bodies have avoided the subspecies conundrum by ignoring subspecies altogether. The authors of a compilation of mammalian taxonomy note that “subspecies have not yet been addressed here due to their inconsistent historical usages in mammalogy, although future efforts are likely to compile subspecies information for the purposes of species-level synonymy.”2“About the Mammal Diversity Database,” Mammal Diversity Database website, accessed May 31, 2022, https://www.mammaldiversity.org/about.html. In ornithology, the committee tasked with providing a taxonomic classification last used subspecies in its 1957 list (AOU 1957); and Zink (2004) and Phillimore and Owens (2006) find that a low percentage of avian subspecies are supported by modern methods of molecular systematics, in support of Remsen’s (2005) sentiments.
Thus, there is no agreed-upon list of subspecies, even for taxonomically well-studied groups such as birds and mammals, because (1) there is no agreed-upon general definition of subspecies and (2) many subspecies have been found to be invalid upon modern reanalysis (see below). Thus, it is easy to understand why the USFWS does not have a consistent set of criteria for subspecies recognition and rather defers to taxonomists in a particular group. We suggest that it should be the purview of taxonomists in different fields to find common ground for defining subspecies to be used in ESA listing decisions.
At Least Two Perceived Uses of Subspecies
The Rio Grande wild turkey is a useful segue to considering what characteristics subspecies ought to possess. As Remsen (2005) notes, many subspecies are arbitrary divisions of clines, and it is easy to partition a cline in different ways, and sometimes different characters have different clines that lead to yet different subspecies limits. Remsen (2005) and Taylor et al. (2017) also make it clear that a “good” subspecies should be a discrete entity. That is, a discrete taxonomic entity should have diagnostic boundaries defined by concordant patterns of morphology or genetics, which would qualify the entities as the basic, or lowest, taxonomic unit. Thus, the owls and thrasher discussed earlier are good examples of qualified subspecies. However, others suggest that subspecies are by definition not discretely differentiated populations or groups of independently evolving populations but have “fuzzy” edges owing either to ongoing introgression (gene flow) or to insufficient time having elapsed since the cessation of genetic exchange (the so-called lag effect). In the former case the evolving unit is geographically larger than each participating subspecies. Nonetheless, this is an oft-cited justification for subspecies: a subspecies designation signifies that “something interesting is going on” that could be of interest to ecologists or evolutionary biologists.
Winker (2010) considers subspecies a gold mine of testable hypotheses in evolutionary biology. Indeed, this can be an important function of subspecies, but such subspecies should not be construed as worthy of conservation status under the ESA—only those that are discretely differentiated should be considered worthy of conservation status. The reason is that otherwise, there will be thousands of such arbitrarily defined subspecies that could be accorded taxonomic trinomials and therefore qualify for listing. For example, the author of the Rio Grande wild turkey subspecies could have erected very different subspecies boundaries, depending on the definition of “intermediate”, and such subspecies could be of interest to evolutionary biologists (to direct study toward understanding the causes of geographic variation) but not to taxonomists.
To be clear, we do not argue against preserving “evolutionarily interesting” parts of species ranges, but—given the need to prioritize support—they should be less important than genuinely evolutionarily distinct units (Taylor et al. 2017). In conservation decisions, the status quo is to accept a subspecies if it has a formal taxonomic name, irrespective of whether its attributes have been tested since its description. This results in the potential to name thousands of arbitrarily defined subspecies, when in fact they are not important elements of biodiversity at the lowest taxonomic level.
Tests of Subspecies
What Constitutes a Strong Test of Subspecies Limits?
A strong test of a listed subspecies, as envisioned by Taylor et al. (2017), would include the comparison of statistically adequate samples from throughout listed subspecies with samples of other subspecies, preferably those geographically adjacent. Listed subspecies should have at least two geographically spaced samples (if possible), allowing a researcher to test whether each sample is more closely related to the other than to samples from other subspecies. There should be no sampling gaps that would give the illusion of real genetic or morphological discontinuities (see Rising 2001) owing simply to geographic distance between sampling localities. Evidence of taxonomic distinctiveness ought to be gathered from several character systems, with preference perhaps given to modern molecular methods. All data must be publicly available, and the analyses must be clearly described. The data should show concordant geographic splits in multiple character systems (Barrowclough 1982), which would confirm a hypothesis of evolutionary independence; it is inappropriate to delete characters with conflicting patterns. This sets a high bar for taxonomic descriptions of subspecies.
Molecular methods have revolutionized tests of subspecies and their evolutionary independence (Avise 1992). The foundation of the ESA rests on the assumption that listed entities are evolutionarily independent. If one examines morphological characters, which are almost certainly under strong selection, one does not expect a single evolutionary history to emerge. The reason is that characters often respond idiosyncratically to opposing environmental dimensions, and therefore picking one morphological character to draw subspecies boundaries leads to a lack of evolutionary consistency. Only when a suite of morphological or genetic characters all show the same pattern can one safely infer that the pattern reflects the history of population subdivision.
Molecular characters used to date are usually considered to be “selectively neutral”—that is, not influenced unduly by natural selection—and hence the only reason for congruent geographic patterns is that they reflect a common underlying evolutionary history. Therefore, if a population or group of populations is evolving independently, one expects correspondence among a host of neutral genetic characters. Patten and Remsen (2017) failed to understand this basic point when they claimed that neutral genetic characters should not be expected to map to subspecies boundaries. The point they missed is that subspecies as described by several generations of morphological taxonomists do not map to evolutionarily independent groupings. Thus, it is not surprising that most subspecies lack molecular genetic support. It is not the genetics that failed; it is the morphological characters that contain ambiguous and conflicting information and fail to recover evolutionary entities.
Is There Such a Thing as Too Much Resolving Power with Modern Genetic Methods?
The El Segundo blue butterfly (Euphilotes battoides allyni) is a federally listed subspecies found along the coast of southern California. Dupuis et al. (2020) used a sophisticated molecular analysis and found that this subspecies is distinct. However, north and south along the coast are six additional, equally distinct genetic clusters. Should all be accorded the same protection? Either there are too few subspecies of E. battoides or the newer techniques will find minor differences of statistical importance irrespective of subspecies boundaries—differences of dubious biological significance.
The federally listed butterfly Lange’s metalmark (Apodemia mormo langei) occupies a narrow range. MtDNA studies found it to be reciprocally monophyletic, supporting its listing (Proshek et al. 2015), although the researchers remark, “Placed in the context of the entire species complex, A. m. langei is no more genetically distinctive than most populations in California, and other populations exist in this region that exhibit higher mitochondrial and nuclear divergence.” In this case, the subspecies framework does not capture the evolutionary pattern. Saglam et al. (2017) used nuclear genomics to test subspecies limits in two trout, Oncorhynchus clarkii seleniris and O. c. henshawi, which were ambiguous with mtDNA. Their analyses found that both subspecies were highly distinctive. Given the potential resolving power of new techniques, it will be possible to find what appear to be local isolates but in fact are the result of isolation by distance (see below). That is, local family groups could give the illusion of genetic gaps. Hence, although the heightened resolving power of next-generation sequencing methods is unprecedented, the results should be interpreted cautiously.
A subspecies of great interest to conservation biologists is the southwestern willow flycatcher (Empidonax traillii extimus). In the only authoritative statement on subspecies in North America, the American Ornithologists’ Union (1957) Checklist, this subspecies was not accepted, although it had been described nine years earlier (Phillips 1948). Data sets on mtDNA, amplified fragment length polymorphisms, niche modeling, and song vocalizations supported the AOU’s decision to not designate E. t. extimus as a valid subspecies (Zink 2015; 2016; see Mahoney et al. 2020). But when Ruegg et al. (2021) analyzed variation in 105,000 single nucleotide polymorphisms (SNPs) from 175 individuals, they concluded that the subspecies was valid.
There are concerns about Ruegg et al.’s (2021) sampling and data analysis, however. For example, they used a principal component analysis to conclude that there were seven “main clusters.” They used these groupings to choose SNPs that best discriminated among these clusters, which borders on a circular procedure. Also, there are sampling gaps between E. t. extimus and the subspecies to the north (E. t. adastus), there are no samples from the southern extent of the range in Mexico (see below), and there is no assessment of isolation by distance. The samples from California, within the range of E. t. extimus, do not group with those in the eastern part of the subspecies’ range. Thus, Ruegg et al. (2021) clearly found geographic differentiation in genetic variation throughout the range of the species, but whether the data recover the limits of E. t. extimus as described by Phillips (1948) is unclear.
Thus, the so-called next-generation sequencing methods need to be interpreted with caution so as not to confuse sampling and genetic gaps (see below) and so as not to cherry-pick SNPs that favor one hypothesis over another. That is, one might exclude characters that suggest a different pattern, whereas overall differentiation should be assessed across all characters (e.g., SNPs). That is, conflicting characters should be a part of the analysis so as not to bias the result to a preconceived conclusion. Needless to say, assigning individuals to preordained subspecies is a flawed procedure.
Economics and Subspecies: The Cost of Invalid Subspecies
Preserving rigorously defined subspecies listed under the ESA is a justifiable cost to society, given the often-stated goal of preserving biodiversity (Leopold 1989). These subspecies have evolved over thousands or tens of thousands of years and represent potentially unique adaptations to their local environments. They are an important part of our biodiversity heritage, and we owe it to future generations to see to their preservation. The listing of arbitrarily defined subspecies, in contrast, serves only to draw resources from species and subspecies that merit protection.
Costs of preservation vary widely within and among different groups of organisms (Gordon et al. 2020). At the level of full species, the average cost of preserving a bird species in the United States is $2,571,017, with a wide range of variation. One of the costliest vertebrate species is the delta smelt (Hypomesus transpacificus), whose annual cost of preservation is about $39 million. According to Gordon et al. (2020), mammals cost 8–26 times more on average to conserve than plants, and bird species cost 5–30 times more to conserve than plants and 6–14 times more than aquatic invertebrates. Bias toward “charismatic” species is also well documented (Courchamp et al. 2018). Thus, spending money on ill-defined taxa is inappropriate.
Preserving subspecies also carries a hefty price tag. The coastal California gnatcatcher (Polioptila californica californica) is listed as threatened. Its range includes the densely populated area of southern California from Palos Verde Peninsula south to the border with Baja California (and farther south to the end of Baja California Sur, where it becomes relatively common). The validity of the subspecies has been challenged (Zink et al. 2000; 2013; 2015) and defended (McCormack and Maley 2015), and recent genomics data show that it is not evolutionarily distinct and hence not a valid subspecies (Vázquez-Miranda et al. 2022). Its listing has prevented the development of coastal sage scrub habitat, and the USFWS has suggested that excluding this habitat has come at a cost of at least $1 billion (Gordon 2018). Fortunately, much of the land occupied by the coastal California gnatcatcher is currently preserved by habitat conservation plans (Winchell and Doherty 2018), and gnatcatcher populations are apparently genetically connected (Vandergast et al. 2019). This subspecies is essentially a symbol for an entire biome: coastal sage scrub. However, there is no current legislation like the ESA for biomes. Obviously, other subspecies occupying less densely populated areas would carry a much lower price tag.
Review of Subspecies Listed under the ESA
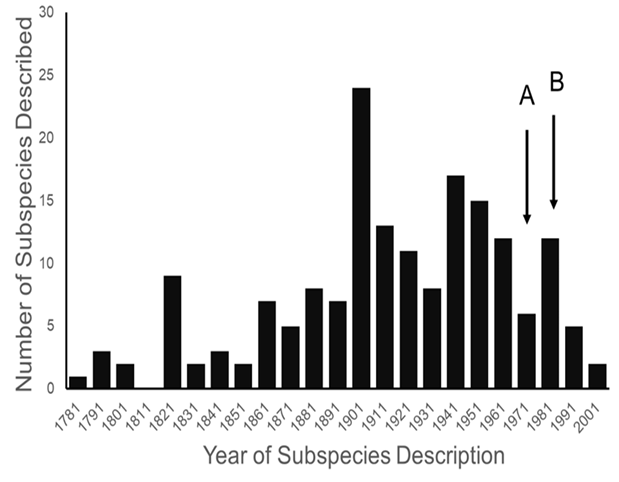
Most ESA-listed subspecies were described before 1950 (137 of 175), and 150 (86%) were described before 1966 (see figure 1), using methods that involved assessments of morphological variation. It was not until 1966 that the first molecular methods appeared that could be used to test subspecies limits. These molecular methods were a major improvement because they allowed the actual genetic basis for subspecies distinctiveness to be measured quantitatively. Knowing the relationship between patterns of actual genetic differentiation and subspecies limits greatly aids efforts to clarify the validity of subspecies.
The molecular methods used evolved from relatively crude assessment of distinguishing alleles at protein-coding loci (allozyme electrophoresis) to studies involving thousands of base-pairs at the DNA level. Most molecular examinations (n = 92) of subspecies limits used mtDNA (n = 67), and some were combinations of mtDNA and microsatellites (n = 19) or mtDNA and nuclear DNA (n = 14). Evaluations of listed subspecies vary widely in their sampling size, from a single individual to over 100 samples. Given the variation in the areal extent of listed subspecies’ distributions, a diversity in sampling size is not surprising; however, the relative percentage of the distribution covered by sampling also varies widely. Clearly, “point samples” are insufficient, and a valid test requires at least two samples within each subspecies so that the relationships within and between subspecies can be assessed. However, gaining permission to acquire the necessary samples of threatened and endangered subspecies to perform modern sequencing methods and close sampling gaps may prove difficult. Although these difficulties should not be ignored, in several cases researchers were able to include only a single population represented by one individual, thus making inferences of population distinctiveness difficult.
Figure 1. Distribution of years in which subspecies listed under the endangered species act were described. Arrow “A” marks the beginning of the use of allozymes in studying genetic variation in natural populations, and arrow “B” marks the beginning of the use of mitochondrial DNA.
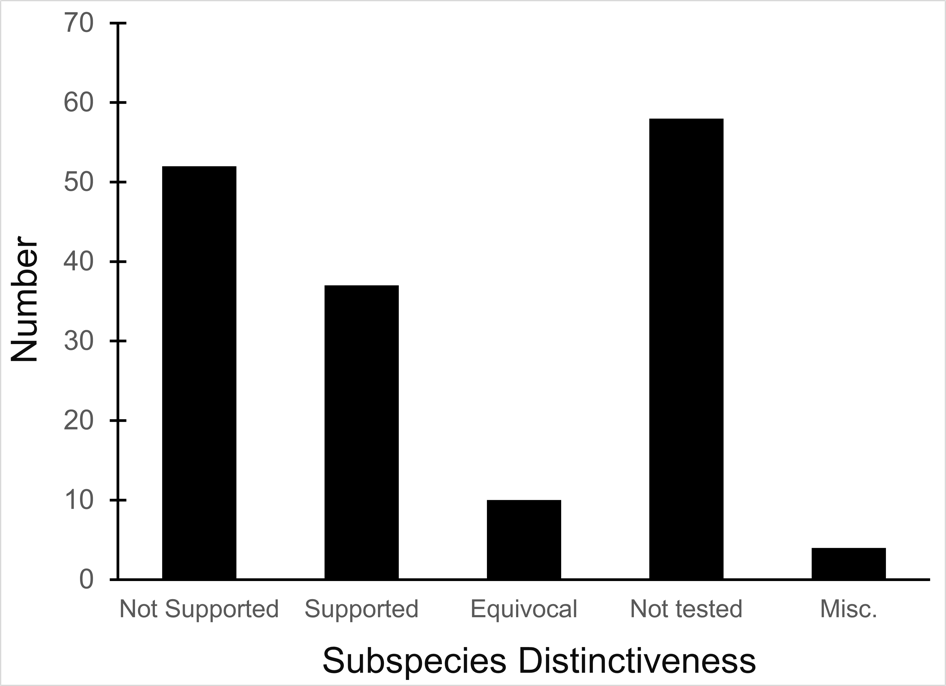
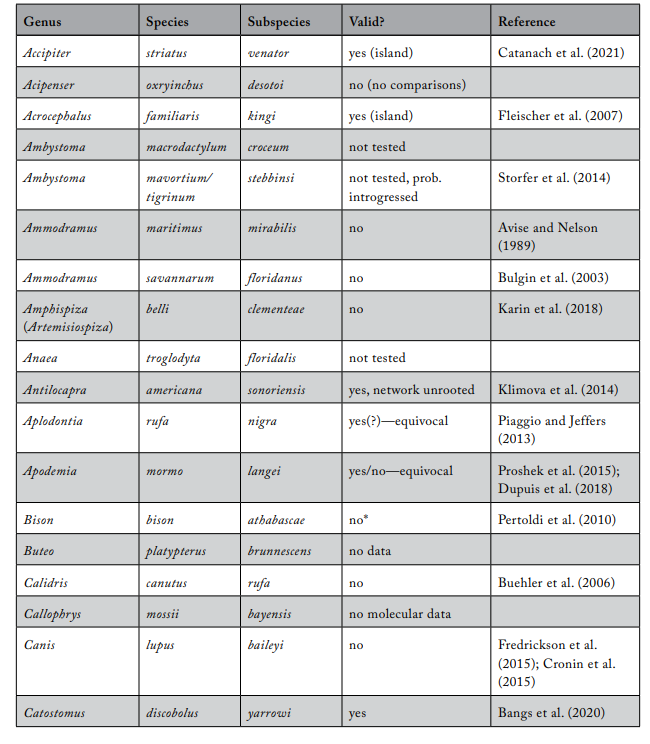
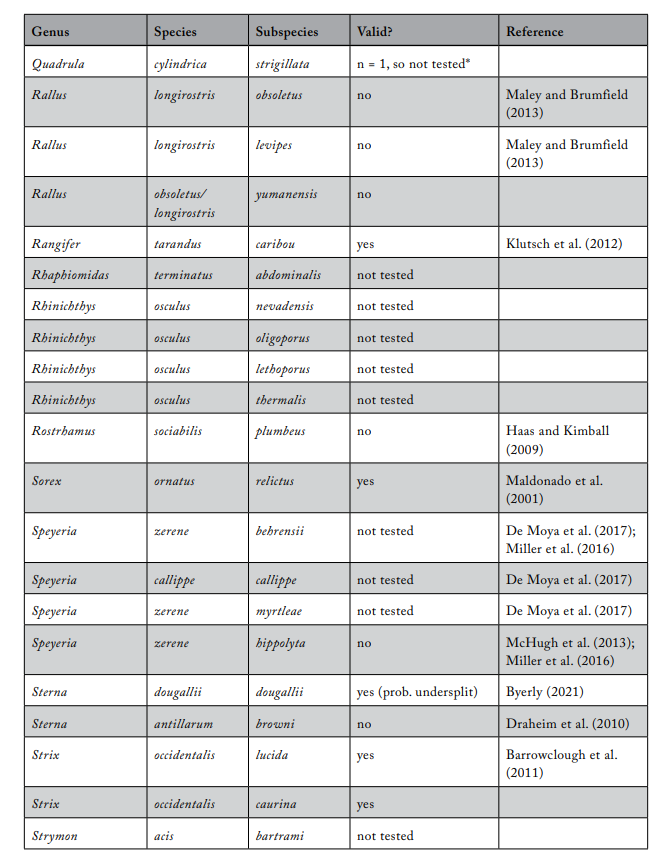
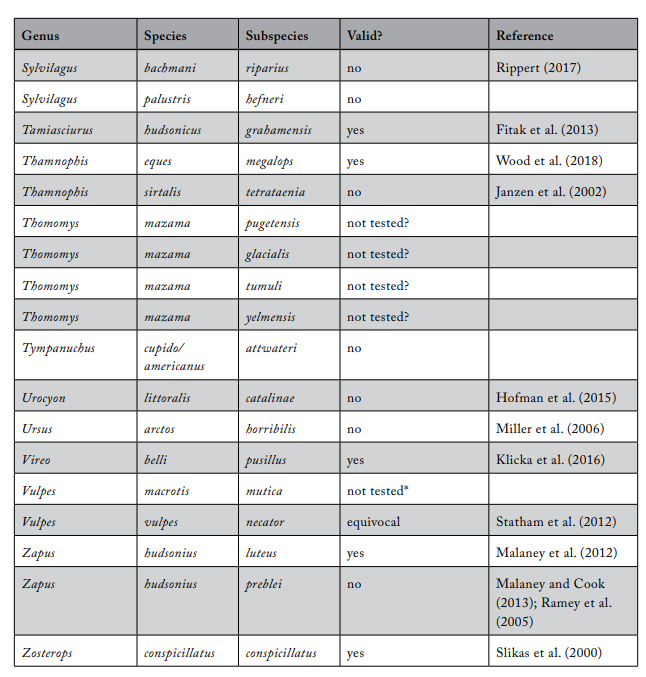
We examined 165 listed subspecies to determine how many were supported by modern analyses (see table 1: 11 have been removed the USFWS in ITIS; https://www.itis.gov/). As noted earlier, a valid test would include multiple samples within the subspecies and comparisons with adjacent samples. Seven subspecies have been removed from the list because, according to the ITIS website, they have been elevated to species or there was a data error in the description. We found that the bulk of the remaining subspecies are distributed relatively equally among three categories: supported, not supported, and not tested (figure 2). This summary suggests that a listed subspecies has a fifty-fifty chance of being significant.
Figure 2. Distribution of results of evaluation of listed vertebrate subspecies. “Not tested” means samples from listed subspecies were not compared with samples from adjacent subspecies, there were too few samples, or samples were not examined.
We found that the ITIS classification rather strongly departs from our summary. More than 40 of the 51 subspecies (78%) that were not supported by our evaluation were considered valid on the ITIS website.
Role of Introductions in Obscuring Historical Units
Tursi et al. (2013) found that the Lower Keys marsh rabbit (Sylvilagus palustris hefneri) was not distinct from other subspecies, and that introductions might be required to maintain populations. They identified areas from which rabbits could be translocated to keep genetic integrity to the greatest degree possible. In many other cases, modern molecular methods are used to choose populations to serve as sources for reintroductions.
What if a narrowly distributed subspecies goes extinct? The San Joaquin woodrat (Neotoma fuscipes riparia) is found in a small area in the Central Valley of California. It is closely related to adjacent populations of different subspecies. If it is determined that the woodrat’s ecological role is important, perhaps individuals from adjacent populations could be transplanted. Granted, this would not reconstitute the genetic integrity of the lost populations, but the transplants might fill the woodrat’s ecological role. Similar examples exist with the dusky seaside sparrow (Ammospiza maritima nigrescens) and the Florida panther (Puma concolor couguar), where preserving the animal’s ecological role at the expense of historical taxonomic integrity appears to be the goal.
Summary and Future Considerations
Caveats
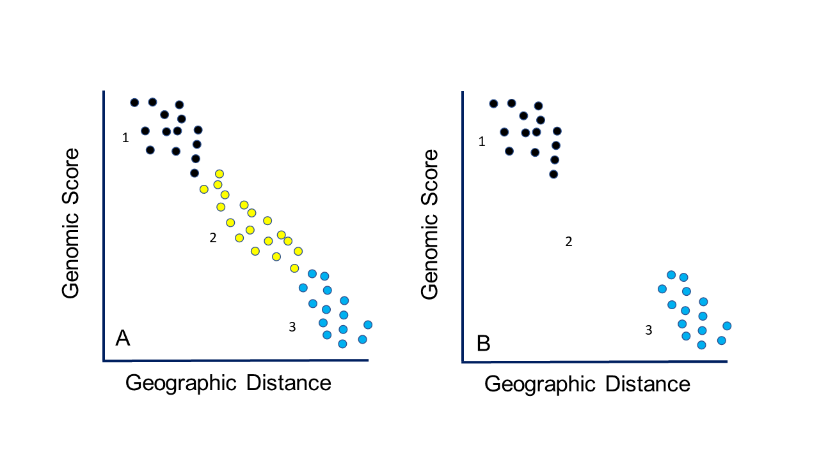
The molecular methods used to test subspecies have evolved greatly over the past few decades, owing to a large increase in resolving power. With the new potential to describe genomes of individuals, some issues should be recognized. First, if sampling is not evenly spaced, sampling gaps will give the illusion of discrete taxonomic boundaries (figure 3). Also, if a gap in the range is caused by anthropogenic elimination of intermediate areas, the populations might appear distinct, albeit not from natural evolutionary processes. Subspecies limits cannot be tested without a clear and rigorous sampling protocol.
It is possible for a subspecies that is not evolutionary distinct (a category that includes many subspecies) to be ecologically important—perhaps important enough to merit listing. Examples might include keystone species such as large carnivores: the Florida panther, for instance. However, providing quantitative data of ecological importance might be as large a task as documenting taxonomic distinctiveness. The lack of consistency among subspecies definitions used in ESA listings is a major failing of taxonomists. To further the use of taxonomic work in conservation decisions, this failing ought to be addressed.
Figure 3. A. Clinal gradient in genetics showing three groups. B. The effects either of not sampling from region 2, and mistaking isolation by distance for discrete groups, or of region 2 being extirpated because of anthropogenic factors, which will give the illusion of discrete groups that could be classified taxonomically.
The Path Forward
Given the high cost of subspecies preservation and the fact that roughly 50% of subspecies tested are supported by modern methods, it should be unacceptable to list a subspecies under the ESA without modern analyses following the criteria given earlier. Taylor et al. (2017) developed a set of quantitative criteria for use in cetacean subspecies taxonomy. These criteria are unlikely to be broadly applicable, but they could form a template for taxonomists of different groups of organisms to work from. Therefore, it should be incumbent upon the USFWS to undertake valid surveys of the listed subspecies to determine whether they are taxonomically distinct and qualified to be evolutionary units under the ESA. That is, it should not be assumed that because a subspecies exists in a checklist, it is evolutionarily significant. In addition, USFWS should seek consensus among taxonomists who work on different groups or organisms to agree on a list of minimal criteria for a subspecies to be listed under the ESA so that listing decisions are transparent.
Table 1. Review of subspecies listed under the US Endangered Species Act
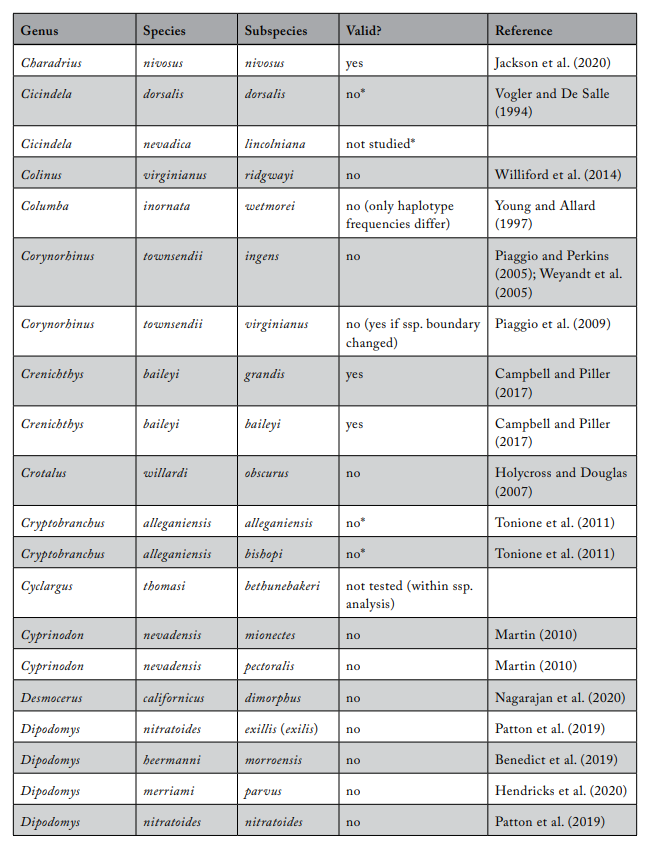
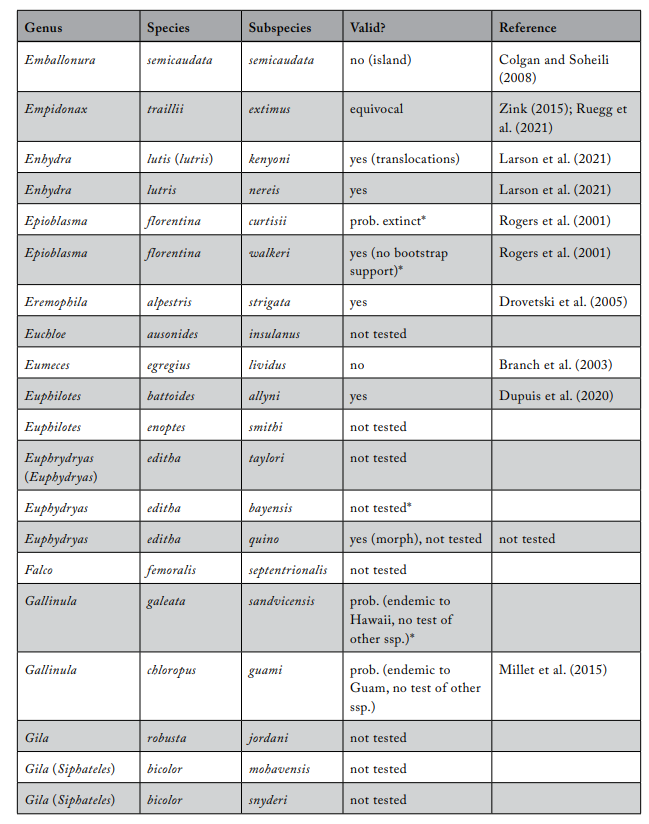
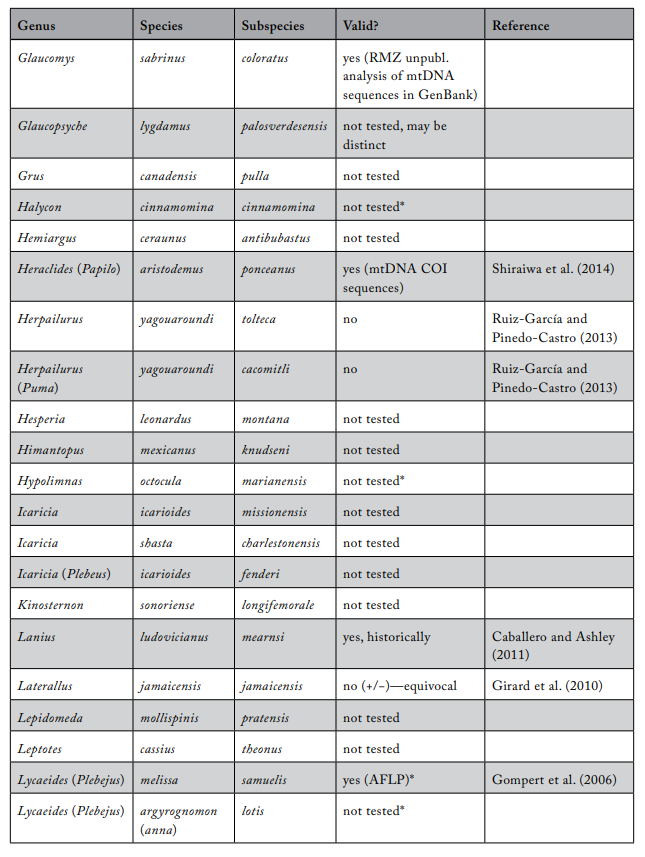
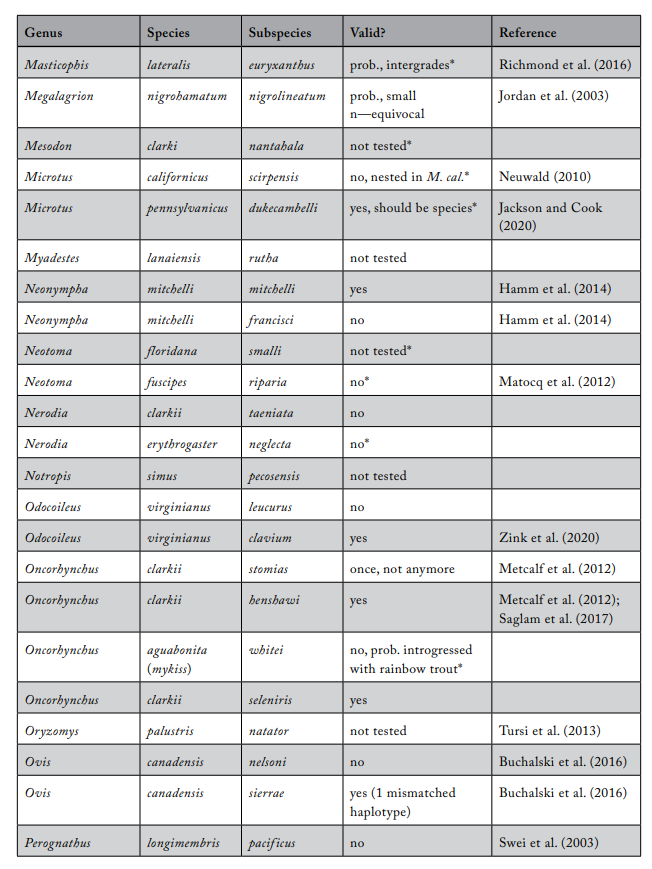
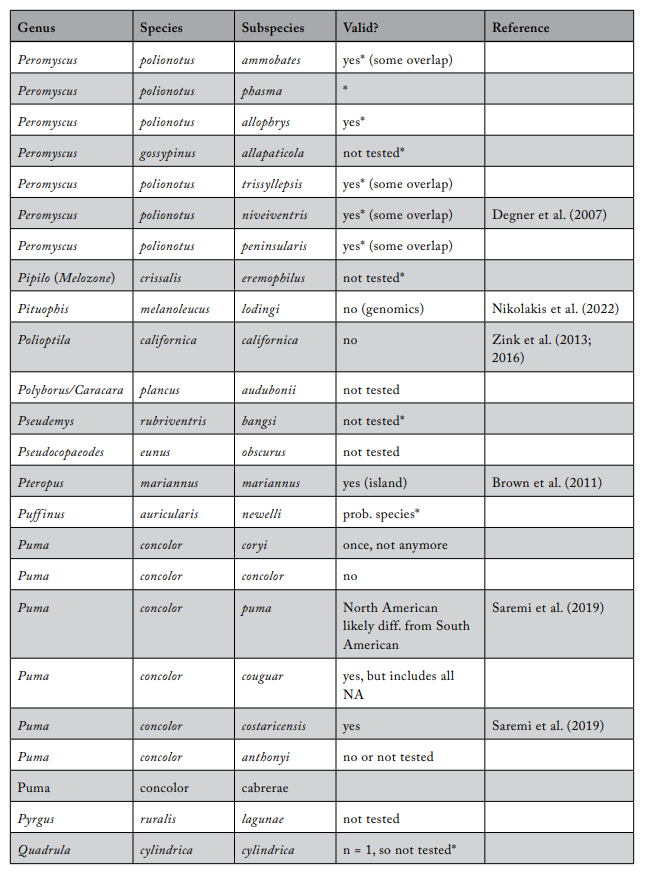
* The Integrated Taxonomic Information System considers the subspecies invalid. (In most cases, the basis for an invalid conclusion is a change in the scientific name of the described subspecies.)
Note: This list does not include other subspecies already considered invalid by the Integrated Taxonomic Information System: Aerodramus vanikorensis bartschi, Bufo hemiophrys baxteri, Drymarchon corais couperi, Epicrates monensis monensis, Epioblasma torulosa gubernaculum, Epioblasma torulosa rangiana, Epioblasma obliquata obliquata, Epioblasma torulosa torulosa, Epioblasma obliquata perobliqua, Epioblasma florentina florentina, Gasterosteus aculeatus williamsoni, Lasiurus cinereus semotus, Oxyloma haydeni kanabensis.
References
American Ornithologists’ Union. 1957. The check-list of North American birds, fifth ed. Baltimore, Maryland, Amer. Ornith. Union.
Arbogast, B. S. (1999). Mitochondrial DNA phylogeography of the New World flying squirrels (Glaucomys): implications for Pleistocene biogeography. Journal of Mammalogy, 80(1), 142–155.
Avise, J. C. (1992). Molecular population structure and the biogeographic history of a regional fauna: a case history with lessons for conservation biology. Oikos, 62–76.
Avise, J. C., Nelson, W. S. (1989). Molecular genetic relationships of the extinct dusky seaside sparrow. Science, 243(4891), 646–648.
Bangs, M. R., Douglas, M. R., Chafin, T. K., Douglas, M. E. (2020). Gene flow and species delimitation in fishes of western North America: flannelmouth (Catostomus latipinnis) and bluehead sucker (C. Pantosteus discobolus). Ecology and Evolution, 10(13), 6477–6493.
Barrowclough, G. F. (1982). Geographic variation, predictiveness, and subspecies. The Auk, 99, 601–603.
Barrowclough, G. F., Cracraft, J., Klicka, J., Zink, R. M. (2016). How many kinds of birds are there and why does it matter? PLoS One, 11(11), e0166307.
Barrowclough, G. F., Groth, J. G., Mertz, L. A., Gutiérrez, R. J. (2006). Genetic structure of Mexican Spotted Owl (Strix occidentalis lucida) populations in a fragmented landscape. The Auk, 123(4), 1090–1102.
Barrowclough, G. F., Gutiérrez, R. J., Groth, J. G., Lai, J. E., Rock, D. F. (2011). The hybrid zone between northern and California spotted owls in the Cascade–Sierran suture zone. The Condor, 113(3), 581–589.
Benedict, B. D., Castellanos, A. A., Light, J. E. (2019). Phylogeographic assessment of the Heermann’s kangaroo rat (Dipodomys heermanni). Journal of Mammalogy, 100(1), 72–91.
Braby, M. F., Eastwood, R., Murray, N. (2012). The subspecies concept in butterflies: has its application in taxonomy and conservation biology outlived its usefulness?. Biological Journal of the Linnean Society, 106(4), 699–716.
Branch, L. C., Clark, A. M., Moler, P. E., Bowen, B. W. (2003). Fragmented landscapes, habitat specificity, and conservation genetics of three lizards in Florida scrub. Conservation Genetics, 4(2), 199–212.
Brown, V. A., Brooke, A., Fordyce, J. A., McCracken, G. F. (2011). Genetic analysis of populations of the threatened bat Pteropus mariannus. Conservation Genetics, 12(4), 933–941.
Buchalski, M. R., Sacks, B. N., Gille, D. A., Penedo, M. C. T., Ernest, H. B., Morrison, S. A., Boyce, W. M. (2016). Phylogeographic and population genetic structure of bighorn sheep (Ovis canadensis) in North American deserts. Journal of Mammalogy, 97(3), 823–838.
Buehler, D. M., Baker, A. J., Piersma, T. (2006). Reconstructing palaeoflyways of the late Pleistocene and early Holocene red knot Calidris canutus. Ardea -Wageningen-, 94(3), 485–498.
Bulgin, N. L., Gibbs, H. L., Vickery, P., Baker, A. J. (2003). Ancestral polymorphisms in genetic markers obscure detection of evolutionarily distinct populations in the endangered Florida grasshopper sparrow (Ammodramus savannarum floridanus). Molecular Ecology, 12(4), 831–844.
Burbrink, F. T., Crother, B. I., Murray C. M., Smith, B. T., Ruane, S., Myers, E. A., Pyron, R. A. Empirical and philosophical problems with the subspecies rank. Ecology and Evolution, in press.
Byerly, P. A. (2021). Ecology and Conservation Genomics of Roseate Terns in North America (Doctoral dissertation, University of Louisiana at Lafayette).
Caballero, I. C., Ashley, M. V. (2011). Genetic analysis of the endemic island loggerhead shrike, Lanius ludovicianus anthonyi. Conservation Genetics, 12(6), 1485–1493.
Campbell, D. C., Piller, K. R. (2017). Let’s jump in: a phylogenetic study of the Great Basin springfishes and poolfishes, Crenichthys and Empetrichthys (Cyprinodontiformes: Goodeidae). PLoS One, 12(10), e0185425.
Catanach, T. A., Halley, M. R., Allen, J. M., Johnson, J. A., Thorstrom, R., Palhano, S., Thunder, C. P., Gallardo, J. C., Weckstein, J. D. (2021). Systematics and conservation of an endemic radiation of Accipiter hawks in the Caribbean islands. The Auk, 138(3), ukab041.
Colgan, D. J., Soheili, S. (2008). Evolutionary lineages in Emballonura and Mosia bats (Mammalia: Microchiroptera) from the southwestern Pacific. Pacific Science, 62(2), 219–232.
Courchamp, F., Jaric, I., Albert, C., Meinard, Y., Ripple, W. J., Chapron, G. (2018). The paradoxical extinction of the most charismatic animals. PLoS Biology, 16(4), p.e2003997.
Cracraft, J. (1983). Species concepts and speciation analysis. In Current ornithology (pp. 159–187). Springer, New York, NY.
Craig, J. M., Crampton, W. G., Albert, J. S. (2017). Revision of the polytypic electric fish Gymnotus carapo (Gymnotiformes, Teleostei), with descriptions of seven subspecies. Zootaxa, 4318(3), 401–438.
Cronin, M. A., Cánovas, A., Bannasch, D. L., Oberbauer, A. M., Medrano, J. F. (2015). Wolf subspecies: reply to Weckworth et al. and Fredrickson et al. Journal of Heredity, 106(4), 417–419.
Degner, J. F., Stout, I. J., Roth, J. D., Parkinson, C. L. (2007). Population genetics and conservation of the threatened southeastern beach mouse (Peromyscus polionotus niveiventris): subspecies and evolutionary units. Conservation Genetics, 8(6), 1441–1452.
De Moya, R. S., Savage, W. K., Tenney, C., Bao, X., Wahlberg, N., Hill, R. I. (2017). Interrelationships and diversification of Argynnis Fabricius and Speyeria Scudder butterflies. Systematic Entomology, 42(4), 635–649.
de Queiroz, K. (2007). Species concepts and species delimitation. Systematic Biology, 56(6), 879–886.
Draheim, H. M., Miller, M. P., Baird, P., Haig, S. M. (2010). Subspecific status and population genetic structure of Least Terns (Sternula antillarum) inferred by mitochondrial DNA control-region sequences and microsatellite DNA. The Auk, 127(4), 807–819.
Drovetski, S. V., Pearson, S. F., Rohwer, S. (2005). Streaked horned lark Eremophila alpestris strigata has distinct mitochondrial DNA. Conservation Genetics, 6(6), 875–883.
Dupuis, J. R., Geib, S. M., Osborne, K. H., Rubinoff, D. (2020). Genomics confirms surprising ecological divergence and isolation in an endangered butterfly. Biodiversity and Conservation, 29(6), 1897–1921.
Dupuis, J. R., Oliver, J. C., Brunet, B. M., Longcore, T., Johnson, J. J., Sperling, F. A. (2018). Genomic data indicate ubiquitous evolutionary distinctiveness among populations of California metalmark butterflies. Conservation Genetics, 19(5), 1097–1108.
Fitak, R. R., Koprowski, J. L., Culver, M. (2013). Severe reduction in genetic variation in a montane isolate: the endangered Mount Graham red squirrel (Tamiasciurus hudsonicus grahamensis). Conservation Genetics, 14(6), 1233–1241.
Fleischer, R. C., Slikas, B., Beadell, J., Atkins, C., Mcintosh, C. E., Conant, S. (2007). Genetic variability and taxonomic status of the Nihoa and Laysan Millerbirds. The Condor, 109(4), 954–962.
Fredrickson, R. J., Hedrick, P. W., Wayne, R. K., vonHoldt, B. M., Phillips, M. K. (2015). Mexican wolves are a valid subspecies and an appropriate conservation target. Journal of Heredity, 106(4), 415–416.
Girard, P., Takekawa, J. Y., Beissinger, S. R. (2010). Uncloaking a cryptic, threatened rail with molecular markers: origins, connectivity and demography of a recently-discovered population. Conservation Genetics, 11(6), 2409–2418.
Gompert, Z., Nice, C. C., Fordyce, J. A., Forister, M. L., Shapiro, A. M. (2006). Identifying units for conservation using molecular systematics: the cautionary tale of the Karner blue butterfly. Molecular Ecology, 15(7), 1759–1768.
Gordon, E. R., Butt, N., Rosner‐Katz, H., Binley, A. D., Bennett, J. R. (2020). Relative costs of conserving threatened species across taxonomic groups. Conservation Biology, 34(1), 276–281.
Gordon, R. (2018). Whatever the cost of the Endangered Species Act, it’s huge. Competitive Enterprise Institute, No. 247. https://cei.org/sites/default/files/Robert_Gordon_-_%E2%80%9CWhatever_the_Cost%E2%80%9D_of_the_Endangered_Species_Act%2C_It%E2%80%99s_Huge.pdf
Haas, S. E., Kimball, R. T. (2009). Genetic divergence among snail kite subspecies: implications for the conservation of the endangered Florida Snail Kite (Rostrhamus sociabilis). Ibis, 151, 181–185.
Haig, S. M., Beever, E. A., Chambers, S. M., Draheim, H. M., Dugger, B. D., Dunham, S., Elliott‐Smith, E., Fontaine, J. B., Kesler, D. C., Knaus, B. J., Lopes, I. F. (2006). Taxonomic considerations in listing subspecies under the US Endangered Species Act. Conservation Biology, 20(6), 1584–1594.
Hamm, C. A., Rademacher, V., Landis, D. A., Williams, B. L. (2014). Conservation genetics and the implication for recovery of the endangered Mitchell’s satyr butterfly, Neonympha mitchellii mitchellii. Journal of Heredity, 105(1), 19–27.
Hendricks, S., Navarro, A. Y., Wang, T., Wilder, A., Ryder, O. A., Shier, D. M. (2020). Patterns of genetic partitioning and gene flow in the endangered San Bernardino kangaroo rat (Dipodomys merriami parvus) and implications for conservation management. Conservation Genetics, 21(5), 819–833.
Hofman, C. A., Rick, T. C., Hawkins, M. T. R., Funk, W. C., Ralls, K., Boser, C. L., Collins, P. W., et al. (2015). Mitochondrial genomes suggest rapid evolution of dwarf California Channel Islands foxes (Urocyon littoralis). PLoS One, 10(2), e0118240.
Holycross, A. T., Douglas, M. E. (2007). Geographic isolation, genetic divergence, and ecological non-exchangeability define ESUs in a threatened Sky-Island rattlesnake. Biological Conservation, 134(1), 142–154.
Jackson, D. J., Cook, J. A. (2020). A precarious future for distinctive peripheral populations of meadow voles (Microtus pennsylvanicus). Journal of Mammalogy, 101(1), 36–51.
Jackson, J. D. U., Bruford, M. W., Székely, T., DaCosta, J. M., Sorenson, M. D., Russo, I. R. M., Maher, K. H., Cruz-López, M., Galindo-Espinosa, D., Palacios, E., De Sucre-Medrano, A. E. (2020). Population differentiation and historical demography of the threatened snowy plover Charadrius nivosus (Cassin, 1858). Conservation Genetics, 21(3), 387–404.
Janzen, F. J., Krenz, J. G., Haselkorn, T. S., Brodie, E. D., Jr., Brodie, E. D., III (2002). Molecular phylogeography of common garter snakes (Thamnophis sirtalis) in western North America: implications for regional historical forces. Molecular Ecology, 11(9), 1739–1751.
Jordan, S., Simon, C., Polhemus, D. (2003). Molecular systematics and adaptive radiation of Hawaii’s endemic damselfly genus Megalagrion (Odonata: Coenagrionidae). Systematic Biology, 52(1), 89–109.
Karin, B. R., Cicero, C., Koo, M. S., Bowie, R. C. (2018). The role of history and ecology as drivers of song divergence in Bell’s and sagebrush sparrows (Artemisiospiza, Aves: Passerellidae). Biological Journal of the Linnean Society, 125(2), 421–440.
Klicka, L. B., Kus, B. E., Title, P. O., Burns, K. J. (2016). Conservation genomics reveals multiple evolutionary units within Bell’s Vireo (Vireo bellii). Conservation Genetics, 17(2), 455–471.
Klimova, A., Munguia-Vega, A., Hoffman, J. I., Culver, M. (2014). Genetic diversity and demography of two endangered captive pronghorn subspecies from the Sonoran Desert. Journal of Mammalogy, 95(6), 1263–1277.
Klütsch, C. F., Manseau, M., Wilson, P. J. (2012). Phylogeographical analysis of mtDNA data indicates postglacial expansion from multiple glacial refugia in woodland caribou (Rangifer tarandus caribou). PLoS One, 7(12), e52661.
Larson, S., Gagne, R. B., Bodkin, J., Murray, M. J., Ralls, K., Bowen, L., Leblois, R., et al. (2021). Translocations maintain genetic diversity and increase connectivity in sea otters, Enhydra lutris. Marine Mammal Science, 37(4), 1475–1497.
Leopold, A. (1989). A Sand County Almanac, and Sketches Here and There. USA: Oxford University Press.
Luo, A., Ling, C., Ho, S. Y., Zhu, C. D. (2018). Comparison of methods for molecular species delimitation across a range of speciation scenarios. Systematic Biology, 67(5), 830–846.
Mahoney, S. M., Reudink, M. W., Pasch, B., Theimer, T. C. (2020). Song but not plumage varies geographically among Willow Flycatcher Empidonax traillii subspecies. Journal of Avian Biology, 51(12).
Malaney, J. L., Cook, J. A. (2013). Using biogeographical history to inform conservation: the case of Preble’s meadow jumping mouse. Molecular Ecology, 22(24), 6000–6017.
Malaney, J. L., Demboski, J. R., Cook, J. A. (2017). Integrative species delimitation of the widespread North American jumping mice (Zapodinae). Molecular Phylogenetics and Evolution, 114, 137–152.
Malaney, J. L., Frey, J. K., Cook, J. A. (2012). The biogeographic legacy of an imperilled taxon provides a foundation for assessing lineage diversification, demography and conservation genetics. Diversity and Distributions, 18(7), 689–703.
Maldonado, J. E., Vilà, C., Wayne, R. K. (2001). Tripartite genetic subdivisions in the ornate shrew (Sorex ornatus). Molecular Ecology, 10(1), 127–147.
Maley, J. M., Brumfield, R. T. (2013). Mitochondrial and next-generation sequence data used to infer phylogenetic relationships and species limits in the Clapper/King Rail complex R. longirostris/R. elegans. The Condor, 115(2), 316–329.
Mallet, J. (2007). Subspecies, semispecies, superspecies. Encyclopedia of Biodiversity, 5, 523–526.
Martin, A. P. (2010). The conservation genetics of Ash Meadows pupfish populations: I. the Warm Springs pupfish Cyprinodon nevadensis pectoralis. Conservation Genetics, 11(5), 1847–1857.
Matocq, M. D., Kelly, P. A., Phillips, S. E., Maldonado, J. E. (2012). Reconstructing the evolutionary history of an endangered subspecies across the changing landscape of the Great Central Valley of California. Molecular Ecology, 21(24), 5918–5933.
Mayr, E., (1970). Populations, species, and evolution: an abridgment of animal species and evolution (Vol. 19). Harvard University Press.
McHugh, A., Bierzychudek, P., Greever, C., Marzulla, T., Van Buskirk, R., Binford, G. (2013). A molecular phylogenetic analysis of Speyeria and its implications for the management of the threatened Speyeria zerene hippolyta. Journal of Insect Conservation, 17(6), 1237–1253.
McCormack, J. E., and J. M. Maley (2015). Interpreting negative results with taxonomic and conservation implications: Another look at the distinctness of coastal California Gnatcatchers. The Auk 132: 380–388.
Metcalf, J. L., Stowell, S. L., Kennedy, C. M., Rogers, K. B., McDonald, D., Epp, J., Keepers, K., Cooper, A., Austin, J. J., Martin, A. P. (2012). Historical stocking data and 19th century DNA reveal human‐induced changes to native diversity and distribution of cutthroat trout. Molecular Ecology, 21(21), 5194–5207.
Miller, C. R., Waits, L. P., Joyce, P. (2006). Phylogeography and mitochondrial diversity of extirpated brown bear (Ursus arctos) populations in the contiguous United States and Mexico. Molecular Ecology, 15(14), 4477–4485.
Miller, M. P., Mullins, T. D., Haig, S. M. (2016). Genetic diversity and population structure in the threatened Oregon silverspot butterfly (Speyeria zerene hippolyta) in western Oregon and northwestern California—Implications for future translocations and the establishment of new populations: US Geological Survey Open-File Report, 2016–1162, http://dx.doi.org/10.3133/ofr20161162.
Miller, M. P., Mullins, T. D., Haig, S. M., Takano, L., Garcia, K. (2015). Genetic structure, diversity, and interisland dispersal in the endangered Mariana Common Moorhen (Gallinula chloropus guami). The Condor, 117(4), 660–669.
Nagarajan, R. P., Goodbla, A., Graves, E., Baerwald, M., Holyoak, M., Schreier, A. (2020). Non-invasive genetic monitoring for the threatened valley elderberry longhorn beetle. PloS One, 15(1), e0227333.
National Academies of Sciences, Engineering, and Medicine (2019). Evaluating the Taxonomic Status of the Mexican Gray Wolf and the Red Wolf. Washington, DC: The National Academies Press. https://doi.org/10.17226/25351.
Neuwald, J. L. (2010). Population isolation exacerbates conservation genetic concerns in the endangered Amargosa vole, Microtus californicus scirpensis. Biological Conservation, 143(9), 2028–2038.
Nikolakis, Z. L., Orton, R. W., Crother, B. I. (2022). Fine‐scale population structure within an Eastern Nearctic snake complex (Pituophis melanoleucus). Zoologica Scripta, 51(2), 133–146.
Patten, M. A., Remsen, J. V., Jr. (2017). Complementary roles of phenotype and genotype in subspecies delimitation. Journal of Heredity, 108(4), 462–464.
Patton, J. L., Williams, D. F., Kelly, P. A., Cypher, B. L., Phillips, S. E. (2019). Geographic variation and evolutionary history of Dipodomys nitratoides (Rodentia: Heteromyidae), a species in severe decline. Journal of Mammalogy, 100(5), 1546–1563.
Pertoldi, C., Tokarska, M., Wójcik, J. M., Kawałko, A., Randi, E., Kristensen, T. N., Loeschcke, V., et al. (2010). Phylogenetic relationships among the European and American bison and seven cattle breeds reconstructed using the BovineSNP50 Illumina genotyping BeadChip. Acta Theriologica, 55(2), 97–108.
Phillimore, A. B., Owens, I. P. (2006). Are subspecies useful in evolutionary and conservation biology?. Proceedings of the Royal Society B: Biological Sciences, 273(1590), 1049–1053.
Phillips, A. R. (1948). Geographic variation in Empidonax traillii. The Auk, 65, 507–514.
Piaggio, A. J., Jeffers, J. (2013). On the edge: A genetic assessment of Aplodontia rufa from the edge of their distribution. Western North American Naturalist, 73(4), 485–496.
Piaggio, A. J., Navo, K. W., Stihler, C. W. (2009). Intraspecific comparison of population structure, genetic diversity, and dispersal among three subspecies of Townsend’s big-eared bats, Corynorhinus townsendii townsendii, C. t. pallescens, and the endangered C. t. virginianus. Conservation Genetics, 10(1), 143–159.
Piaggio, A. J., Perkins, S. L. (2005). Molecular phylogeny of North American long-eared bats (Vespertilionidae: Corynorhinus): inter- and intraspecific relationships inferred from mitochondrial and nuclear DNA sequences. Molecular Phylogenetics and Evolution, 37(3), 762–775.
Proshek, B., Dupuis, J. R., Engberg, A., Davenport, K., Opler, P. A., Powell, J. A., Sperling, F. A. (2015). Genetic evaluation of the evolutionary distinctness of a federally endangered butterfly, Lange’s metalmark. BMC Evolutionary Biology, 15(1), 1–15.
Ramey, R. R., Liu, H. P., Epps, C. W., Carpenter, L. M., Wehausen, J. D. (2005). Genetic relatedness of the Preble’s meadow jumping mouse (Zapus hudsonius preblei) to nearby subspecies of Z. hudsonius as inferred from variation in cranial morphology, mitochondrial DNA and microsatellite DNA: implications for taxonomy and conservation. Animal Conservation Forum, 8(3), 329–346.
Remsen, J. V., Jr. (2005). Pattern, process, and rigor meet classification. The Auk, 122(2), 403–413.
Richmond, J. Q., Wood, D. A., Swaim, K. E., Fisher, R. N., Vandergast, A. G. (2016). Historical habitat barriers prevent ring-like genetic continuity throughout the distribution of threatened Alameda striped racers (Coluber lateralis euryxanthus). Herpetologica, 72(3), 202–213.
Rippert, J. S. (2017). Population Genetics and Functional Connectivity of the Riparian Brush Rabbit (Sylvilagus bachmani riparius): Implications for the Conservation of an Endangered Lagomorph (Doctoral dissertation, University of Nevada, Reno).
Rising, J. D. (2001). Geographic variation in size and shape of Savannah sparrows (Passerculus sandwichensis). Studies in Avian Biology, 23, 1–65.
Rogers, S. O., Watson, B. T., Neves, R. J. (2001). Life history and population biology of the endangered tan riffleshell (Epioblasma florentina walkeri) (Bivalvia: Unionidae). Journal of the North American Benthological Society, 20(4), 582–594.
Ruegg, K., Anderson, E. C., Somveille, M., Bay, R. A., Whitfield, M., Paxton, E. H., Smith, T. B. (2021). Linking climate niches across seasons to assess population vulnerability in a migratory bird. Global Change Biology, 27(15), 3519–3531.
Ruiz-García, M., Pinedo-Castro, M. (2013). Population genetics and phylogeographic analyses of the jaguarundi (Puma yagouaroundi) by means of three mitochondrial markers: the first molecular population study of this species. Molecular Population Genetics, Phylogenetics, Evolutionary Biology and Conservation of the Neotropical Carnivores. New York: Nova Science Publishers, 245–288.
Saglam, I. K., Prince, D. J., Meek, M., Ali, O. A., Miller, M. R., Peacock, M., Neville, H., et al. (2017). Genomic analysis reveals genetic distinctiveness of the Paiute cutthroat trout Oncorhynchus clarkii seleniris. Transactions of the American Fisheries Society, 146(6), 1291–1302.
Saremi, N. F., Supple, M. A., Byrne, A., Cahill, J. A., Coutinho, L. L., Dalén, L., Figueiró, H. V., et al. (2019). Puma genomes from North and South America provide insights into the genomic consequences of inbreeding. Nature Communications, 10(1), 1–10.
Sennett, G. B. (1879). Further notes on the ornithology of the Lower Grande of Texas, from observations made during the spring of 1878. Bulletin of the United States Geographical Survey, 5, 371–440.
Shiraiwa, K., Cong, Q., Grishin, N. V. (2014). A new Heraclides swallowtail (Lepidoptera, Papilionidae) from North America is recognized by the pattern on its neck. Zookeys, 468, 85.
Slikas, B., Jones, I. B., Derrickson, S. R., Fleischer, R. C. (2000). Phylogenetic relationships of Micronesian White-eyes based on mitochondrial sequence data. The Auk, 117(2), 355–365.
Spomer, S. M. (2004). A new subspecies of Cicindela nevadica LeConte (Coleoptera: Carabidae: Cicindelinae) from the badlands of South Dakota. The Coleopterists Bulletin, 58(3), 409–412.
Statham, M. J., Rich, A. R., Lisius, S. K., Sacks, B. N. (2012). Discovery of a remnant population of Sierra Nevada red fox (Vulpes vulpes necator). Northwest Science, 86, 122–132.
Storfer, A., Mech, S. G., Reudink, M. W., Lew, K. (2014). Inbreeding and strong population subdivision in an endangered salamander. Conservation Genetics, 15(1), 137–151.
Swei, A., Brylski, P. V., Spencer, W. D., Dodd, S. C., Patton, J. L. (2003). Hierarchical genetic structure in fragmented populations of the little pocket mouse (Perognathus longimembris) in southern California. Conservation Genetics, 4(4), 501–514.
Taylor, B.L., Perrin, W.F., Reeves, R.R., Rosel, P.E., Wang, J.Y., Cipriano, F., Scott Baker, C. and Brownell Jr, R.L. (2017). Why we should develop guidelines and quantitative standards for using genetic data to delimit subspecies for data‐poor organisms like cetaceans. Marine Mammal Science, 33(S1), 12–26.
Tonione, M., Johnson, J. R., Routman, E. J. (2011). Microsatellite analysis supports mitochondrial phylogeography of the hellbender (Cryptobranchus alleganiensis). Genetica, 139(2), 209–219.
Tursi, R. M., Hughes, P. T., Hoffman, E. A. (2013). Taxonomy versus phylogeny: evolutionary history of marsh rabbits without hopping to conclusions. Diversity and Distributions, 19(2), 120–133.
Vandergast, A. G., Kus, B. E., Preston, K. L., Barr, K. R. (2019). Distinguishing recent dispersal from historical genetic connectivity in the coastal California gnatcatcher. Scientific Reports, 9(1), 1–12.
Vázquez‐Miranda, H., Griffin, J.A., Sheppard, J.M., Herman, J.M., Rojas‐Soto, O. and Zink, R.M., 2017. Morphological and molecular evolution and their consequences for conservation and taxonomy in the Le Conte’s Thrasher Toxostoma lecontei. Journal of Avian Biology, 48(7) 941–954.
Vázquez-Miranda, H., Zink, R. M., and Pinto, B. J. (2022). Comparative phylogenomic patterns in the Baja California avifauna, their conservation implications, and the stages in lineage divergence. Molecular Phylogenetics and Evolution, 171, 107466.
Villanova, V. (2015). Genetic Structure and Demographic Analysis of Key Deer (Odocoileus virginianus clavium). Electronic Theses and Dissertations, 2004–2019. 1435. https://stars.library.ucf.edu/etd/1435.
Vogler, A. P., De Salle, R. (1994). Mitochondrial DNA evolution and the application of the phylogenetic species concept in the Cicindela dorsalis complex (Coleoptera: Cicindelidae). Carabid Beetles: Ecology and Evolution, Springer Dordrecht, 79–85.
Weyandt, S. E., van den Bussche, R., Hamilton, M. J., Leslie, D. M. (2005). Unraveling the effects of sex and dispersal: conservation genetics of the endangered Ozark big-eared bat (Corynorhinus townsendii ingens). Journal of Mammalogy, 85, 140–148.
Williford, D., Deyoung, R. W., Honeycutt, R. L., Brennan, L. A., Hernández, F., Wehland, E. M., Sands, J. P., Demaso, S. J., Miller, K. S., Perez, R. M. (2014). Contemporary genetic structure of the northern bobwhite west of the Mississippi River. The Journal of Wildlife Management, 78(5), 914–929.
Wilson, E. O., Brown, W. L., Jr. (1953). The subspecies concept and its taxonomic application. Systematic Zoology, 2, 97–111.
Winchell, C. S., Doherty, P. F., Jr. (2018). Restoring habitat for coastal California gnatcatchers (Polioptila californica californica). The Condor, 120(3), 581–595.
Winker, K. (2010). Subspecies represent geographically partitioned variation, a gold mine of evolutionary biology, and a challenge for conservation. Ornithological Monographs, 67(1), 6–23.
Wood, D. A., Emmons, I. D., Nowak, E. M., Christman, B. L., Holycross, A. T., Vandergast, A. G. (2018). Conservation genomics of the Mogollon narrow-headed gartersnake (Thamnophis rufipunctatus) and northern Mexican gartersnake (Thamnophis eques megalops): US Geological Survey Open-File Report, 2018–1141, https://doi.org/10.3133/ofr20181141.
Young, D. L., Allard, M. W. (1997). Conservation genetics of the plain pigeon Columba inornata in Puerto Rico and the Dominican Republic. Molecular Ecology, 6(9), 877–879.
Zink, R. M. (1997). Species concepts. Bulletin of the British Ornithologists’ Club, 117, 97–109.
Zink, R. M. (2004). The role of subspecies in obscuring avian biological diversity and misleading conservation policy. Proceedings of the Royal Society of London. Series B: Biological Sciences, 271(1539), 561–564.
Zink, R. M. (2015). Genetics, morphology, and ecological niche modeling do not support the subspecies status of the endangered Southwestern Willow Flycatcher (Empidonax traillii extimus). The Condor, 117(1), 76–86.
Zink, R. M. (2016). Current topics in avian conservation genetics with special reference to the Southwestern Willow Flycatcher. Open Ornithology Journal, 9, 60–69. https://doi.org/10.2174/1874453201609010060.
Zink, R. M., G. F. Barrowclough, J. L. Atwood, and R. C. Blackwell (2000). Genetics, taxonomy and conservation of the threatened California Gnatcatcher. Conservation Biology 14:1394–1405.
Zink, R. M., J. G. Groth, H. Vázquez-Miranda, and G. F. Barrowclough (2013). Phylogeography of the California Gnatcatcher (Polioptila californica) using multilocus DNA sequences and ecological niche modeling: implications for conservation. The Auk 130: 449–458
Zink, R. M., Groth, J. G., Vázquez-Miranda, H., Barrowclough, G. F. (2016). Geographic variation, null hypotheses and subspecies limits in the California Gnatcatcher: A response to McCormack and Maley. The Auk, 133, 59–68.
Zink, R. M., Najar, N., Vázquez-Miranda, H., Buchanan, B. L., Loy, D., Brodersen, B. W. (2020). Geographic variation in the PRNP gene and its promoter, and their relationship to chronic wasting disease in North American deer. Prion, 14(1), 185–192.