
Without a vaccine for COVID-19, the pandemic will be here for some time. Measures such as social distancing and robust surveillance can control the spread of the disease, but there will always be a risk that a flare-up will take lives, especially those of elderly and immunocompromised individuals. A return to normalcy depends on the availability of a vaccine, or possibly of several vaccines, as the virus continues to mutate over time.
America needs to return to normal as soon as possible, for the sake of the economy if not people’s sanity. Unfortunately, the FDA approval process is not likely to result in a marketable vaccine until sometime next year. To resolve this mismatch in timelines, Congress should create an expedited process to allow patients, via a process of informed consent, to use vaccine candidates that have not yet completed the full FDA approval process.
Good News and Bad News about Vaccines
Meeting the need for a vaccine is a “good news, bad news” proposition. The good news is that creating vaccines has never been easier. Thanks to modern science, scientists have everadvancing tools and more possible approaches than ever before. In addition to traditional whole-pathogen approaches, scientists today can make subunit vaccines as well as state-ofthe-art DNA and RNA vaccines. Synthetic biology is being used as a design tool to make these vaccines more effective than ever.1Sharon Begley, “To Develop a Coronavirus Vaccine, Synthetic Biologists Try to Outdo Nature,” STAT, March 9, 2020. San Diego–based Inovio Pharmaceuticals claims to have designed a DNA vaccine candidate for COVID-19 only three hours after the publication of the SARS-CoV-2 genome.2Inovio, “Inovio Accelerates Timeline for COVID-19 DNA Vaccine INO-4800,” press release, March 3, 2020, http://ir.inovio.com/news-and-media/news/press-release-details/2020/Inovio-Accelerates-Timeline-for-COVID-19-DNA-Vaccine-INO-4800/default.aspx.
The bad news is that vaccine trials for new diseases take a long time. “The traditional vaccine timeline is 15 to 20 years,” says Mark Feinberg, president and CEO of the International AIDS Vaccine Initiative.3Eric Boodman, “Coronavirus Vaccine Clinical Trial Starting without Usual Animal Data,” STAT, March 11, 2020 Given the urgency of addressing the COVID-19 pandemic, the world is moving faster: for example, researchers have skipped the usual animal testing of Moderna’s mRNA vaccine candidate and have already begun human clinical trials in Seattle.4Boodman, “Coronoavirus Vaccine Clinical Trial”; Denise Grady, “Trial of Coronavirus Vaccine Made by Moderna Begins in Seattle,” New York Times, March 16, 2020. Even so, experts say it will take between a year and 18 months to get an FDA-approved COVID-19 vaccine on the market. This means that even if society “flattens the curve” and gets some respite from warm summer weather, the population will still be unprotected in the fall, when colder weather could be expected to drive an increase in COVID-19 contagion.
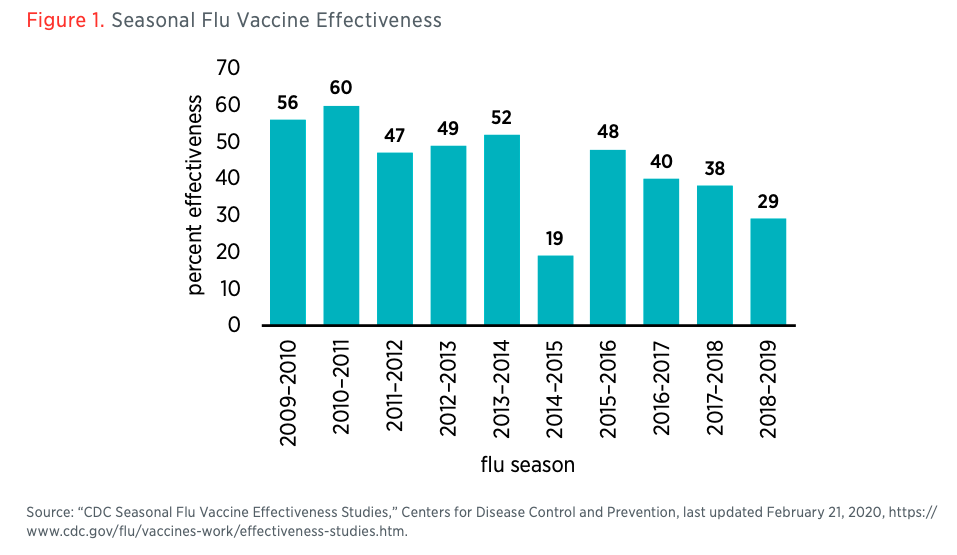
Safety and effectiveness of vaccines should be thoroughly studied, but in this extreme situation all should recognize that safety and effectiveness are not all-or-nothing properties. A vaccine that is available this summer that is probably (but not guaranteed to be) safe could be better than no vaccine going into a second wave of the pandemic this fall. And effectiveness even for approved vaccines is known to be incomplete and variable. According to the Centers for Disease Control and Prevention, the flu vaccine for the 2018–2019 flu season was only 29 percent effective (see figure 1).5“CDC Seasonal Flu Vaccine Effectiveness Studies,” Centers for Disease Control and Prevention, last updated February 21, 2020, https://www.cdc.gov/flu/vaccines-work/effectiveness-studies.htm. That said, even a partially effective vaccine improves herd immunity and drives the effective reproduction rate of the virus down so that outbreaks are easier to contain. The seasonal flu is a challenging target, and there is every reason to expect that a coronavirus vaccine could have higher effectiveness, but even partial effectiveness creates significant value.
Risks and Benefits of Early Access to Vaccine Candidates
To be sure, letting Americans use vaccine candidates entails some risk. Even approved vaccines can have negative effects. The dengue vaccine worsens the illness in patients who have not had dengue before, a phenomenon known as vaccine-induced enhancement.6Jon Cohen, “New Dengue Vaccine Performs Well in Large Trial, but Safety Remains Key Concern,” Science, November 6, 2019. An H1N1 vaccine that was administered to 25 percent of the US population in 1976 caused an increase in Guillain-Barré syndrome.7Rebecca Kreston, “The Public Health Legacy of the 1976 Swine Flu Outbreak,” Discover, September 30, 2013. And an approved 2009 H1N1 vaccine appears to have caused an outbreak of narcolepsy in Europe.8“Narcolepsy Following Pandemrix Influenza Vaccination in Europe,” Centers for Disease Control and Prevention, last updated January 29, 2020, https://www.cdc.gov/vaccinesafety/concerns/history/narcolepsy-flu.html.
Early vaccine candidates for SARS, a disease closely related to COVID-19, had to be pulled for vaccine-induced enhancement.9Mike Hixenbaugh, “Scientists Were Close to a Coronavirus Vaccine Years Ago. Then the Money Dried Up,” NBC News, March 5, 2020. The world still doesn’t have a SARS vaccine, but there is reasonable hope that progress against a related virus will transfer and that at least some of the COVID-19 vaccines will work.
People already accept risks of vaccine candidates to the informed and consenting human subjects who volunteer for clinical trials. Simply allowing additional informed and consenting subjects to take similar risks does not pose new ethical quandaries. And early data from the trials will make the public’s decisions about whether to be inoculated more informed and less risky than those of the trial subjects.
Additionally, some might fear that giving the public access to unproven vaccine candidates could further harm trust in vaccines in those segments of the population that already mistrust them. This need not be the case. The FDA could loudly trumpet the fact that some vaccines that are being administered with patient consent have not been approved by the usual process. The media could make clear that there is no approved vaccine for COVID-19 and that those getting an experimental vaccine candidate would be going outside the usual process.
Against these risks, there is the opportunity for significant benefits. More subjects trying these experimental vaccines means more data. A higher N yields greater statistical significance. Researchers can know more sooner about whether the vaccines work, and it’s possible that they can even certify the vaccines sooner with this additional data.
Most fundamentally, America has the opportunity to avoid another large outbreak in the fall. If it seems that at least some of the vaccine trials are going well by June or July, it’s possible that large numbers of people would accept the risk of using an unapproved vaccine. America can avoid the weeks of self-isolation and a Q4 economic crash like the one it is having now. By combining even partially effective immunity from the vaccine with acquired immunity from patients who have recovered from COVID-19, society can reduce the effective reproduction rate of the virus. This could result in hundreds or thousands of saved lives, and it could also avoid large amounts of economic disruption.
What Congress Should Do
To make this possibility viable, Congress should instruct the FDA to publish vaccine trial data in real time. The public should be kept aware of the number of subjects who have received the vaccine candidate, subjects’ demographic information, what the dosages were, whether there were any complications and what they were, whether follow-up testing was able to assess immunity, and whether any subjects became infected.
In addition, Congress should extend liability protections to COVID-19 vaccine candidate manufacturers. Sections 300aa–22 and 300aa–23 of title 42 of the US Code provide some liability protection for vaccine manufacturers, but only for those products that have complied with all requirements of the Federal Food, Drug, and Cosmetic Act, including the requirement to have received an approval from FDA. Manufacturers would be unwilling to supply vaccine candidates if they did not receive a similar waiver of liability for unapproved vaccines. Liability protection should be offered only on the condition that the manufacturer does not withhold information or engage in any kind of fraud or other criminal activity related to the vaccine candidate, similar to the limitations that exist on the legal immunity for approved vaccines.
Finally, Congress will need to create the mechanism by which patients can opt to forgo the protections of vaccine approval based on informed consent. This provision is what would give Americans the right to try the vaccine candidate based on their and their doctors’ assessment of the risks and benefits. The doctrine of informed consent is a cornerstone of modern medical ethics, and the right to use unapproved vaccine candidates follows directly from it.10Jessica Flanigan, “Respect Patients’ Choices to Self-Medicate,” Cato Unbound, July 10, 2017.
The COVID-19 pandemic is a crisis. In the United States, existing approval procedures appear to have caused a botched rollout of testing for the disease. It would be tragic to repeat this mistake with respect to vaccines. Very soon, some data with respect to the safety and effectiveness of vaccine candidates will be available. Going into a possible fall resurgence, America’s only hope for a vaccine is to let people act based on the best information they have. It would be foolish to withhold potentially lifesaving vaccines from the American public during this crisis on the grounds that researchers and regulators cannot deviate from the usual approval practices.
View the pdf here.


 SSRN
SSRN Mercatus Center
Mercatus Center